Lanthanum is the first element found in the lanthanide series, it was discovered in 1839. Its name is derived from a verb in the Greek language whose meaning is hidden, because this metal was hidden in a cerium ore.
This element was discovered in impure cerium nitrate, extracted from lanthanum soil and treated with a strong acid. There are other elements of this class that have been discovered in impurities of minerals belonging to cerium and yttrium.
Contents
Electron configuration of lanthanum

In the case of lanthanum, its simplified or abbreviated electron configuration is [Xe]5d16s2. Its complete electron configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 5d1 6s2.
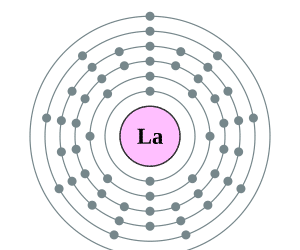
As lanthanum is composed of 57 electrons in total, the distribution of its configuration is as follows: the first shell houses 2 electrons, the second shell has 8, the third shell is composed of 18 electrons, in the fourth shell there are has 18 electrons too, in the fifth shell there are 9 electrons and the sixth has two.
Lanthanum is located in period 6 and group 3 of the periodic table of chemical elements. The covalent radius of this element is 169 pm and its average radius is equal to 195 pm.
The atomic mass of lanthanum is 138.9055 u. To know the atomic mass of an element, you need to know the total mass of protons and neutrons present in a single atom that is part of that element.
Lanthanum Chemical Properties
Lanthanum is in the group of lanthanides, these lanthanides are also known as rare earths because they are usually found in the form of oxides. As for the actinides, the lanthanides are the elements characterized by an internal transition.
The natural state of this element is solid. Its chemical symbol is La and its corresponding atomic number is 57. This chemical element has a silvery-white hue.
The boiling point of this element is equal to 3457.85 degrees Celsius or degrees Celsius, equal to 3730 degrees Kelvin. While the melting point is 920.85 degrees Centigrade or Celsius, similar to 1193 Kelvin.
Lanthanum isotopes
The natural lanthanum represented by La57 is composed of a radioactive isotope La138 and a stable isotope La139, the latter being the one with the highest natural abundance, representing 99.91%.
There are 38 characterized radioisotopes and the most stable is La138, which has a half-life of 102 × 109 years. La137 has a decay period of 60,000 years and La140 has a half-life of approximately 1.6781 days.
The other radioactive isotopes are composed of half-lives of less than a day, almost all less than a minute. Lanthanum is also composed of 12 nuclear isomers, the longest being La132m which has a half-life equal to 24.3 minutes.
Uses of Lanthanum
- Lanthanum oxide is used in glass to provide base strength and to create specialty optical glasses.
- When lanthanum is alloyed with cerium, praseodymium, neodymium, ytterbium, or gadolinium, it creates the alloy called mischmetal. This is used to create lighter stones.
- It is useful as a compound for intensifying screens in x-ray units.
- Hydrogen sponges are made with alloys composed of lanthanum. These alloys pass 400 times the volume of their gas and this procedure is reversible. When they use the gas, thermal energy is released. For this reason, they can be transformed into energy efficient systems.
- Lanthanum carbonate is used to treat chronic renal failure due to the ability to form insoluble complexes with phosphates, thereby minimizing hyperphosphatemia.
Health and environmental effects of lanthanum
Lanthanum is dangerous because it can be inhaled through the air and can cause pulmonary embolism, especially when taken for a long time. This element can cause liver damage by accumulating in the human body.
In the case of aquatic animals, lanthanum damages the membrane cells, which has negative consequences on the functions of the nervous system and on reproduction.