Sebopeho sa elektronike se ngotsoe ka ho fumana lielektrone tsohle tsa athomo kapa ion ho orbitals ea tsona kapa li-sublevels tsa matla.
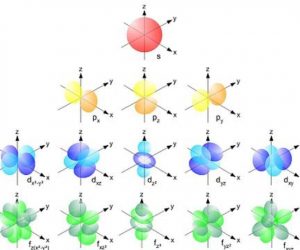
Hopola hore ho na le matla a 7 a matla: 1, 2, 3, 4, 5, 6 le 7. 'Me e' ngoe le e 'ngoe ea tsona e na le mekhahlelo e 4 ea matla e bitsoang s, p, d le f.
Kahoo, boemo ba 1 bo na le sublevel s feela; boemo ba 2 bo na le li-sublevels tsa syp; mothati wa 3 o na le methatinyana s, p le d; mme methati ya 4 ho isa ho ya 7 e na le maemo a manyane s, p, d le f.
Sebopeho sa elektronike

Ho bala kabo ea lielektrone maemong a fapaneng a matla, tlhophiso ea Electron e nka linomoro tsa quantum joalo ka tšupiso kapa e li sebelisa feela bakeng sa kabo. Linomoro tsena li re lumella ho hlalosa maemo a matla a lielektrone kapa elektrone e le 'ngoe, hape li hlalosa sebopeho sa li-orbitals tseo e li bonang ha ho ajoa lielektrone sebakeng.
Lethathamo la Tlhophiso ea Element
| Lebitso la Element | letšoao | Nomoro ea Atomic | Boikarabello ba motlakase |
|---|---|---|---|
| Actinium | [Ac] | 89 | 1.1 |
| aluminium | [Al] | 13 | 1.61 |
| Amerika | [Am] | 95 | 1.3 |
| Antimony | [Sb] | 51 | 2.05 |
| argon | [Ar] | 18 | |
| Arsenic | [As] | 33 | 2.18 |
| Astatine | [At] | 85 | 2.2 |
| Barium | [Ba] | 56 | 0.89 |
| Berkelium | [Bk] | 97 | 1.3 |
| Beryllium | [Be] | 4 | 1.57 |
| Bismuth | [Bi] | 83 | 2.02 |
| Bohrium | [Bh] | 107 | |
| Boron | [B] | 5 | 2.04 |
| Bromine | [Br] | 35 | 2.96 |
| Cadmium | [Cd] | 48 | 1.69 |
| khalsiamo | [Ca] | 20 | 1 |
| California | [Cf] | 98 | 1.3 |
| k'habone | [C] | 6 | 2.55 |
| Cerium | [Ce] | 58 | 1.12 |
| cesium | [Cs] | 55 | 0.79 |
| Chlorine | [Cl] | 17 | 3.16 |
| Chromium | [Cr] | 24 | 1.66 |
| Cobalt | [Co] | 27 | 1.88 |
| koporo | [Cu] | 29 | 1.9 |
| Curium | [Cm] | 96 | 1.3 |
| Darmstadtium | [Ds] | 110 | |
| Dubnium | [Db] | 105 | |
| Dysprosium | [Dy] | 66 | 1.22 |
| einsteinium | [Es] | 99 | 1.3 |
| Erbium | [Er] | 68 | 1.24 |
| Europium | [Eu] | 63 | |
| fermium | [Fm] | 100 | 1.3 |
| Fluorine | [F] | 9 | 3.98 |
| Foramo | [Fr] | 87 | 0.7 |
| Gadolinium | [Gd] | 64 | 1.2 |
| Gallium | [Ga] | 31 | 1.81 |
| Germanium | [Ge] | 32 | 2.01 |
| Gold | [Au] | 79 | 2.54 |
| Hafnium | [Hf] | 72 | 1.3 |
| Hassium | [Hs] | 108 | |
| Helium | [He] | 2 | |
| Holmium | [Ho] | 67 | 1.23 |
| Hydrogen | [H] | 1 | 2.2 |
| India | [In] | 49 | 1.78 |
| Iodine | [I] | 53 | 2.66 |
| Iridium | [Ir] | 77 | 2.2 |
| tšepe | [Fe] | 26 | 1.83 |
| krypton | [Kr] | 36 | 3 |
| Lanthanum | [La] | 57 | 1.1 |
| Lawrencium | [Lr] | 103 | |
| loto | [Pb] | 82 | 2.33 |
| Lithium | [Li] | 3 | 0.98 |
| Lutetium | [Lu] | 71 | 1.27 |
| magnesium | [Mg] | 12 | 1.31 |
| Manganese | [Mn] | 25 | 1.55 |
| Meitnerium | [Mt] | 109 | |
| Mendelevium | [Md] | 101 | 1.3 |
| Mercury | [Hg] | 80 | 2 |
| Molybdenum | [Mo] | 42 | 2.16 |
| Neodymium | [Nd] | 60 | 1.14 |
| Neon | [Ne] | 10 | |
| Neptunium | [Np] | 93 | 1.36 |
| nikele | [Ni] | 28 | 1.91 |
| Niobium | [Nb] | 41 | 1.6 |
| Nitrojene | [N] | 7 | 3.04 |
| Nobelium | [No] | 102 | 1.3 |
| Oganesson | [Uuo] | 118 | |
| Osmium | [Os] | 76 | 2.2 |
| Oxyjene | [O] | 8 | 3.44 |
| Palladium | [Pd] | 46 | 2.2 |
| phosphorus | [P] | 15 | 2.19 |
| Platinum | [Pt] | 78 | 2.28 |
| Plutonium | [Pu] | 94 | 1.28 |
| Polonium | [Po] | 84 | 2 |
| Potassium | [K] | 19 | 0.82 |
| Praseodymium | [Pr] | 59 | 1.13 |
| Prformum | [Pm] | 61 | |
| Protactinium | [Pa] | 91 | 1.5 |
| radium | [Ra] | 88 | 0.9 |
| Radon | [Rn] | 86 | |
| Rhenium | [Re] | 75 | 1.9 |
| Rhodium | [Rh] | 45 | 2.28 |
| Roentgenium | [Rg] | 111 | |
| Rubidium | [Rb] | 37 | 0.82 |
| Ruthenium | [Ru] | 44 | 2.2 |
| Rutherfordium | [Rf] | 104 | |
| Samarium | [Sm] | 62 | 1.17 |
| scandium | [Sc] | 21 | 1.36 |
| Seaborgium | [Sg] | 106 | |
| Selenium | [Se] | 34 | 2.55 |
| Silicone | [Si] | 14 | 1.9 |
| Silver | [Ag] | 47 | 1.93 |
| Sodium | [Na] | 11 | 0.93 |
| Strontium | [Sr] | 38 | 0.95 |
| Sebabole | [S] | 16 | 2.58 |
| Tantalum | [Ta] | 73 | 1.5 |
| Theknoloji | [Tc] | 43 | 1.9 |
| Tellurium | [Te] | 52 | 2.1 |
| Terbium | [Tb] | 65 | |
| Thallium | [Tl] | 81 | 1.62 |
| Thorium | [Th] | 90 | 1.3 |
| Thulium | [Tm] | 69 | 1.25 |
| Tin | [Sn] | 50 | 1.96 |
| thaethaniamo | [Ti] | 22 | 1.54 |
| Tungsten | [W] | 74 | 2.36 |
| Ununbium | [Uub] | 112 | |
| Ununhexium | [Uuh] | 116 | |
| Ununpentium | [Uup] | 115 | |
| Unuquadium | [Uuq] | 114 | |
| Ununseptium | [Uus] | 117 | |
| Ununtrium | [Uut] | 113 | |
| Uranium | [U] | 92 | 1.38 |
| Vanadium | [V] | 23 | 1.63 |
| Xenon | [Xe] | 54 | 2.6 |
| Khutso | [Yb] | 70 | |
| Yttrium | [Y] | 39 | 1.22 |
| zinki | [Zn] | 30 | 1.65 |
| Zirconium | [Zr] | 40 | 1.33 |
Lintlha tseo ho buisanoeng ka tsona ka ho fetisisa!

Ka lebaka la tlhophiso ea Electron, hoa khoneha ho theha thepa ea motsoako ho tloha sebakeng sa lik'hemik'hale tsa liathomo, ka lebaka la sena, ke hore sebaka se lumellanang le sona tafoleng ea periodic se tsejoa. Phetoho ena e bontša tatellano ea elektrone e 'ngoe le e' ngoe maemong a fapaneng a matla, ke hore lipotolohong, kapa e bonts'a feela kabo ea bona ho potoloha khubu ea athomo.
Ke hobane'ng ha tlhophiso ea elektronike e le ea bohlokoa?

Ha elektrone e le hole ho tloha khubung, boemo bona ba matla bo tla ba bo phahameng. Ha lielektrone li le boemong bo tšoanang ba matla, boemo bona bo nka lebitso la orbitals ea matla. U ka hlahloba tlhophiso ea Electron ea likarolo tsohle u sebelisa tafole e hlahang ka holim'a mongolo ona oa thuto.
Tlhophiso ea Electron ea likarolo e boetse e sebelisa nomoro ea athomo ea element e fumanoang tafoleng ea periodic. Hoa hlokahala ho tseba hore na elektronike ke eng, e le hore u ithute sehlooho sena sa bohlokoa ka ho qaqileng.
Boitsebiso bona bo etsoa ka lebaka la linomoro tse 'nè tsa quantum tseo elektrone e' ngoe le e 'ngoe e nang le tsona, e leng:
- nomoro ea bongata ba makenete: e bonts'a mokhoa oa orbital oo elektrone e leng ho oona.
- nomoro ea mantlha ea bongata: ke boemo ba matla boo elektrone e leng ho bona.
- Spin nomoro ea quantum: e bua ka ho potoloha ha elektronike.
- Azimuthal kapa nomoro ea bobeli ea quantum: ke tsela eo elektrone e leng ho eona.
Lipheo tsa tlhophiso ea Electron.
Sepheo se seholo sa tlhophiso ea elektronike ke ho hlakisa tatellano le kabo ea matla ea liathomo, haholo-holo kabo ea boemo bo bong le bo bong ba matla le sublevel.
Mefuta ea tlhophiso ea Electron.
- Tlhophiso ea mantlha.

- Phetoho e atolositsoeng. Ka lebaka la tlhophiso ena, e 'ngoe le e' ngoe ea lielektrone tsa athomo e emeloa e sebelisa metsu ho emela ho potoloha ha e 'ngoe le e 'ngoe. Tabeng ena, ho tlatsoa ho etsoa ho nahanoa ka molao oa Hund oa bongata bo bongata le molao-motheo oa Pauli oa ho khetholla.
- tlhophiso e koalehileng. Maemo 'ohle a tlatsitsoeng ka mokhoa o tloaelehileng a emeloa ke khase e khabane, moo ho nang le ngollano pakeng tsa palo ea athomo ea khase le palo ea lielektrone tse tletseng boemo ba ho qetela. Likhase tsena tse khabane ke: He, Ar, Ne, Kr, Rn le Xe.
- Sebopeho se atolositsoeng ka halofo. Ke motsoako pakeng tsa tlhophiso e atolositsoeng le tlhophiso e khutsufalitsoeng. Ho eona, ke li-electrone feela tsa matla a ho qetela a matla a emeloa.
Lintlha tsa bohlokoa tsa ho ngola tlhophiso ea elektrone ea athomo.
- U tlameha ho tseba palo ea lielektrone tseo athomo e nang le tsona, hobane u tlameha feela ho tseba nomoro ea eona ea athomo kaha sena se lekana le palo ea lielektrone.
- Beha lielektrone boemong bo bong le bo bong ba matla, ho qala ka e haufi.
- Hlompha boholo ba bokhoni ba boemo bo bong le bo bong.
Mehato ea ho fumana tlhophiso ea electron ea element

Tabeng ena, nomoro ea athomo e tafoleng ea periodic e lula e bontšoa ka lebokoseng le ka holimo le letona, ka mohlala, tabeng ea hydrogen, e tla ba palo ea 1 e bonoang karolong e ka holimo ea lebokose lena, ha boima ba eona ba athomo. kapa nomoro ea masico, ke e kentsoeng karolong e ka holimo empa ka lehlakoreng le letšehali.
Tšebeliso ea nomoro ena ea athomo e etsa hore tlhophiso ea eona e khethoe ka tšebeliso ea linomoro tsa quantum le kabo e fapaneng ea lielektrone ho orbit.
Mehlala e meng ea tlhophiso ea element ke ena.
- Hydrogen, nomoro ea eona ea athomo ke 1, ke hore Z=1, ka hona, Z=1:1sa .
- Potassium, nomoro ea eona ea athomo ke 19, kahoo Z=19: 1stsa bona2stsa bona2P63stsa bona3p64stsa bona3dleshome4pa.
Phatlalatso ea elektrone.
E lumellana le kabo ea e 'ngoe le e' ngoe ea lielektrone ho orbitals le maemo a tlase a athomo. Mona tlhophiso ea Electron ea likarolo tsena e laoloa ke setšoantšo sa Moeller.
Bakeng sa ho tseba kabo ea Electron ea element ka 'ngoe, ke linoto feela tse tlamehang ho ngoloa ka mokhoa o ts'oanang ho tloha holimo ho ea tlase le ho tloha ho le letona ho ea ho le letšehali.
Tlhophiso ea likarolo ho latela tlhophiso ea Electron.
Lintho tsohle tsa lik'hemik'hale li arotsoe ka lihlopha tse 'nè, e leng:
- likhase tse khabane. Ba phethile potoloho ea bona ea elektrone ka lielektrone tse robeli, ho sa baloe Eena, e nang le lielektrone tse peli.
- likarolo tsa phetoho. Li na le litsela tse peli tsa ho qetela tse sa fellang.
- Lintho tse ka hare tsa phetoho. Tsena li na le litsela tsa tsona tse tharo tsa ho qetela tse sa fellang.
- moemeli. Tsena li na le potoloho e kantle e sa fellang.
Ho sebetsana le lintlha le metsoako
Ka lebaka la tlhophiso ea Electron ea likarolo, hoa khoneha ho tseba palo ea lielektrone tseo liathomo li nang le tsona meleng ea tsona, e leng molemo haholo ha ho hahoa li-ionic, likamano tse kopanetsoeng le ho tseba lielektrone tsa valence, sena sa ho qetela se lumellana le palo ea li-electrone. hore athomo ya elemente e itseng e na le potolohong ya yona ya ho qetela kapa kgaketla.
Desnity of Elements
All matter has mass and volume., Leha ho le joalo boima ba lintho tse fapaneng bo nka mefuta e fapaneng.